PharmaShots Weekly Snapshots (August 26 – August 30, 2024)
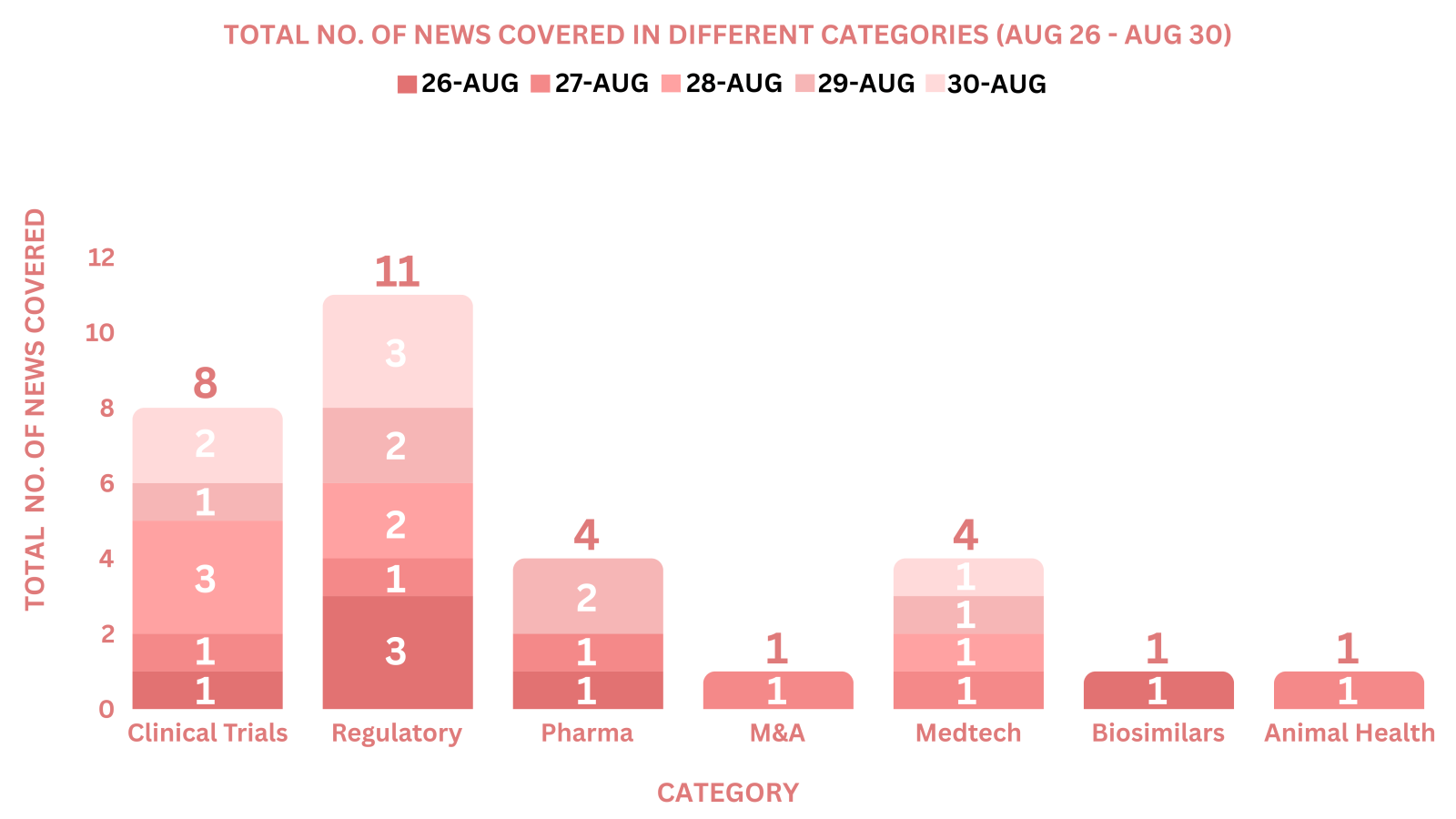
This week PharmaShots’ news was all about the updates on Clinical Trials, Pharma, Biotech, COVID-19, Regulatory & MedTech. Check out our full report below:

Opna Bio Doses the First Patient with OPN-6602 in P-I Trial of Multiple Myeloma
Read More: Opna Bio
Astellas Begins the P-III Clinical Evaluation of Fezolinetant for Vasomotor Symptoms (VMS) Among Breast Cancer Patients
Read More: Astellas
Merck Begins the P-III Clinical Evaluation of Bomedemstat to Treat Essential Thrombocythemia
Read More: Merck
Novartis Reports Topline Data from P-III (V-MONO) Trial of Leqvio (Inclisiran) in Patients at Atherosclerotic Cardiovascular Disease (ASCVD) Risk
Read More: Novartis
Astellas’ Padcev Plus Keytruda Receives the EC’s Approval to Treat Advanced Urothelial Cancer
Read More: Astellas
Merck Discontinues P-III (KEYNOTE-867 and KEYNOTE-630) Studies Evaluating Keytruda for Cancer Treatment
Read More: Merck
Bayer Reports the Initiation of P-III (SOHO-02) Study of BAY 2927088 to Treat Non-Small Cell Lung Cancer (NSCLC)
Read More: Bayer
Gain Therapeutics Reports Topline Data from P-I Study of GT-02287 to Treat Parkinson’s Disease
Read More: Gain Therapeutics

Moderna’s mRESVIA (mRNA-1345) Vaccine Gains the EC’s Approval to Prevent Lower Respiratory Tract Disease (LTRD)
Read More: Moderna
Johnson & Johnson’s Balversa (Erdafitinib) Receives the EC’s Approval to Treat Urothelial Carcinoma
Read More: Johnson & Johnson
Regeneron’s Ordspono (Odronextamab) Receives the EC’s Approval to Treat R/R Follicular Lymphoma and Diffuse Large B-cell Lymphoma
Read More: Regeneron
Roche’s PiaSky Receives the EC’s Approval to Treat Paroxysmal Nocturnal Haemoglobinuria (PNH)
Read More: Roche
GSK’s Nucala (Mepolizumab) Receives Japanese Approval for Treating Chronic Rhinosinusitis with Nasal Polyps
Read More: GSK
CAMP4 Therapeutics’ CMP-CPS-001 Receives the US FDA’s Rare Pediatric Disease Designation to Treat Urea Cycle Disorders
Read More: CAMP4 Therapeutics
GSK’s Arexvy Vaccine Receives the EC’s Approval for its Expanded Age Indication to Prevent Lower Respiratory Tract Disease (LRTD)
Read More: GSK
Life Molecular Imaging’s [18F] PI-2620 Gains the US FDA’s Fast Track Designation for PET Imaging in Various Neurodegenerative Disorders
Read More: Life Molecular Imaging
Johnson & Johnson Reports Nipocalimab’s BLA Submission to the US FDA for Treating Generalized Myasthenia Gravi
Read More: Johnson & Johnson
Median Technologies Reports Results from the REALITY Trial of Eyonis LCS for Lung Cancer Diagnosis
Read More: Median Technologies
Nuvectis Pharma’s NXP800 Receives the US FDA’s Orphan Drug Designation to Treat Various ARID1a-Deficient Cancers
Read More: Nuvectis Pharma

Siemens Healthineers to Acquire Novartis’ Radiodiagnostic Manufacturing and Distribution Network
Read More: Siemens Healthineers & Novartis

Celltrion’s SteQeyma (Biosimilar, Stelara) Receives the EC’s Approval to Treat Various Chronic Inflammatory Diseases
Read More: Celltrion

GI Innovation Join Forces with Merck to Evaluate GI-102 Plus Keytruda for Treating Various Cancers
Read More: GI Innovation
YolTech Therapeutics Partners with Salubris Pharmaceuticals to Develop and Commercialize YOLT-101 in Mainland China
Read More: YolTech Therapeutics & Salubris Pharmaceuticals
Bayer Partners with NextRNA Therapeutics to Develop Small Molecules Targeting Long Non-Coding RNAs (lncRNAs) for Oncology
Read More: Bayer & NextRNA Therapeutics
Lindy Biosciences Teams Up with Novartis for Multi-Target Drug Delivery Innovation
Read More: Lindy Biosciences & Novartis

Insulet Corporation Reports the US FDA’s Clearance of Omnipod 5 Automated Insulin Delivery System for Type 2 Diabetes
Read More: Insulat Corporation
Boston Scientific Receives CE Mark for its ACURATE Prime Aortic Valve System
Read More: Boston Scientific
Neuros Medical Reports the US FDA’s Approval of Altius Direct Electrical Nerve Stimulation System
Read More: Neuros Medical

Merck Animal Health Reports the Accessibility of Autogenous Vaccine for Avian Metapneumovirus Type B
Read More: Merck Animal Health
Related Post: PharmaShots Weekly Snapshots (August 20 – August 23, 2024)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.